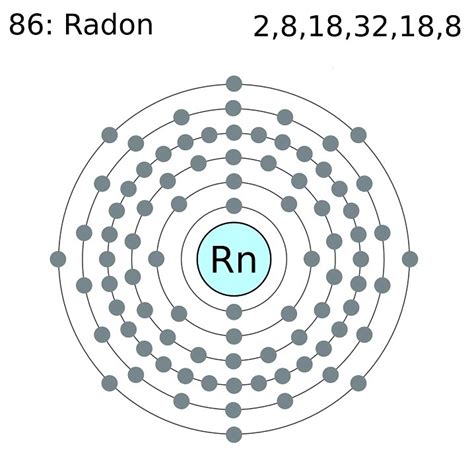
electron configuration for radon|How to write the electron configuration for Radon (Rn) : Manila The electron configuration of radon is [ Xe] 4f 14 5d 10 6s 2 6p 6 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Do you need to convert Central European Time (CET) to India Standard Time (IST) for your travel, business, or personal needs? Use this easy-to-use, modern time zone converter to quickly compare the time difference between CET and IST. You can also explore other time zones and locations with World Time Buddy, the ultimate tool for time travelers.
electron configuration for radon,The electron configuration of radon is [ Xe] 4f 14 5d 10 6s 2 6p 6 , if the electron arrangement is through orbitals. Electron configuration can be done in two ways.
A step-by-step description of how to write the electron configuration for Radon (Rn). In order to write the Rn electron configuration we first need to know the number of e .more
Electronic configuration of the Radon atom. Valence electrons. Orbital diagram.The atomic number of each element increases by one, reading from left to right. BlockElements are organised into blocks by the orbital type in which the outer electrons .

Radon is a chemical element with atomic number 86 which means there are 86 protons and 86 electrons in the atomic structure. The chemical symbol for Radon is .Radon is the 86th element in the periodic table and has a symbol of Rn and atomic number of 86. It has an atomic weight of (222) and a mass number of 193. Radon has eighty-six .
Electron configuration of Radon. The Electron configuration of Radon is Xe 4f14 5d10 6s2 6p6. Radon is a chemical element which is represented by the symbol Rn and whose . Electron configuration: The arrangement of electrons in an atom or molecule. Melting point: The temperature at which a solid substance turns into a liquid. Boiling point: The temperature at which a .
Electron Configuration. [Xe] 4f 14 5d 10 6s 2 6p 6. Rn. Upon condensation, radon glows because of the intense radiation it produces. Physical Properties. Fluorine Electron Configuration. Neon Electron Configuration. The radon is also the immediate decay product of radium. Its most stable 222 Rn, isotope, has a half-life of only 3.8 days which .Rn, 86, radon : 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 Og, 118, . The construction of the periodic table ignores these irregularities and is based on ideal electron configurations. [2] Note the non-linear shell ordering, which comes about due to the different energies of smaller and larger shells . Radon Condensed Electron Configuration. Radon condensed electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6. It is the same as the ground state configuration, with the electrons following all the rules. See also Sulfur Electron Configuration:5 Easy Step-by-Step Guide!Electron configuration 4f 14 5d 10 6s 2 6p 6: Electrons per shell: 2, 8, 18, 32, 18, 8: Physical properties; . Radon is a chemical element; it has symbol Rn and atomic number 86. It is a radioactive noble gas and is .In Electronic Configuration electrons are arranged in various shells, Subshell and Orbital by following certain rules. To Learn how to Write Electronic Configurations, Detailed Explanation, Filling of orbital with FAQs, Visit BYJU’S for detailed explanation. . Radon (Rn) – [Xe]4f 14 5d 10 6s 2 6p 6; Q5 . What is the electronic .How to write the electron configuration for Radon (Rn) Similarly, the observed electron configuration of copper is [Ar] 4s 1 3d 10 instead of [Ar] s 2 3d 9. The actual electron configuration may be rationalized in terms of an added stability associated with a half-filled (ns 1, np 3, nd 5, nf 7) or filled (ns 2, np 6, nd 10, nf 14) subshell. Given the small differences between higher energy levels .Electrons and Electron Configuration. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Radon is 86. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative .

Radon is the 86th element in the periodic table and has a symbol of Rn and atomic number of 86. It has an atomic weight of (222) and a mass number of 193. Radon has eighty-six protons and one hundred seven neutrons in its nucleus, and eighty-six electrons in six shells. It is located in group eighteen, period six and block p of the periodic table.When the 6p orbitals are finally filled, we have reached the next noble gas, radon (Z = 86), [Xe]6s 2 4f 14 5d 10 6p 6 = [Rn]. In the last row, the 5f orbitals are filled between the 7s and the 6d orbitals, which gives the 14 actinide elements. Because the large number of protons makes their nuclei unstable, all the actinides are radioactive .Abbreviated electronic configuration of Radon. The ground state abbreviated electronic configuration of Neutral Radon atom is [Xe] 4f14 5d10 6s2 6p6. The portion of Radon configuration that is equivalent to the noble gas of the preceding period, is abbreviated as [Xe]. For atoms with many electrons, this notation can become lengthy and so an .electron configuration for radon How to write the electron configuration for Radon (Rn) First Ionization Energy of Radon. First Ionization Energy of Radon is 10.7485 eV. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom.. X + energy → .
The electron configurations and orbital diagrams of these four elements are: Figure \(\PageIndex{5}\): Since the core electron shells correspond to noble gas electron configurations, we can abbreviate electron configurations by writing the noble gas that matches the core electron configuration, along with the valence electrons in .Electron Configuration: 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 f 14 5s 2 p 6 d 10 6s 2 p 6; Electrons per Energy Level: 2,8,18,32,18,8 Shell Model; Ionic Radius: . Radon - Rn (EnvironmentalChemistry.com)- Comprehensive information for the element Radon - Rn is provided by this page including scores of properties, element names in many .electron configuration for radon Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, and tasteless gas that is the heaviest known gas and the sixth-heaviest known element. . Electron configuration. The electron configuration of an element describes the arrangement of electrons in the atoms of that element, and be .
Radon (Rn) has an atomic mass of 86. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. ChemicalAid. . Electron Configuration [Xe] 4f 14 5d 10 6s 2 6p 6: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 2 6p 6: Orbital Diagram. Nuclear. Radioactive:Configuración electrónica del radón. La configuración electrónica del radón es 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s2 6p6 Sumando todos estos índices, el total es 86, que es el número total de electrones que tiene el radón, que es igual a su número atómico . How do you write the noble-gas electron configuration for radon? Chemistry Electron Configuration Electron Configuration. 1 Answer mrpauller.weebly.com Oct 3, 2016 [Xe] #6s^2 4f^14 5d^10 6p^6# Explanation: The trick is .Electron configuration: [Xe] 4f 14 5d 10 6s 2 6p 6: Density @ 20 o C: 0.00973 g/cm 3: Show more, including: Heats, Energies, Oxidation, Reactions, Compounds, Radii, Conductivities. Atomic volume: 50.5 cm 3 /mol : Structure: – . Radon gas was discovered in 1900 by Fredrich E. Dorn in Halle, Germany. He described it as radium emanation .
electron configuration for radon|How to write the electron configuration for Radon (Rn)
PH0 · Radon – Electron Configuration and Oxidation States – Rn
PH1 · Radon (Rn)
PH2 · Radon
PH3 · How to write the electron configuration for Radon (Rn)
PH4 · How to write the electron configuration for Radon (Rn
PH5 · How To Find A Electron Configuration For Radon {Rn}
PH6 · Electron configuration of Radon 【Electron Configuration】 2022
PH7 · Electron configuration for Radon (element 86). Orbital diagram
PH8 · Complete Electron Configuration of Radon (Rn)